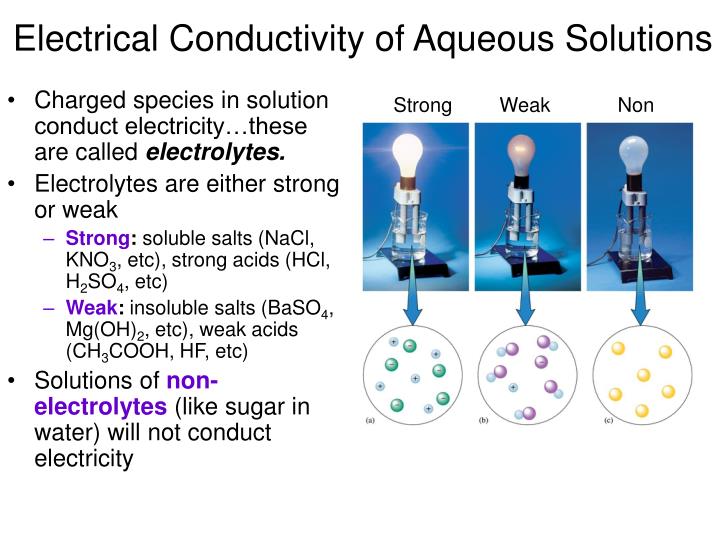
Good Electrical Conductors When Dissolved In Water . A substance that, when added to water, renders it. 4.2.2.8 metals as conductors. Salt contains nacl and kcl, which form electrolytes when dissolved in water, most of which become ions. These compounds are \emph{molecular} and are not acids or bases. The water molecules play an essential part in forming ions. when we dissolve hydrogen chloride gas in water, we find that the solution is a very good conductor. Most familiar is the conduction of electricity. Metals are good conductors of electricity because the delocalised electrons in the metal carry electrical charge through the metal. electrolytic solutions are those that are capable of conducting an electric current. to conduct electricity, a substance must contain freely mobile, charged species. conductivity measurements reveal that most covalent compounds, if they dissolve in water at all, retain their original molecular structures. Substances that dissolve in water to yield ions are called electrolytes. Electrolytes may be covalent compounds that chemically react with water to produce ions (for.
from www.slideserve.com
These compounds are \emph{molecular} and are not acids or bases. Salt contains nacl and kcl, which form electrolytes when dissolved in water, most of which become ions. conductivity measurements reveal that most covalent compounds, if they dissolve in water at all, retain their original molecular structures. Metals are good conductors of electricity because the delocalised electrons in the metal carry electrical charge through the metal. electrolytic solutions are those that are capable of conducting an electric current. when we dissolve hydrogen chloride gas in water, we find that the solution is a very good conductor. 4.2.2.8 metals as conductors. Substances that dissolve in water to yield ions are called electrolytes. A substance that, when added to water, renders it. Most familiar is the conduction of electricity.
PPT Plan for Wed, 1 Oct 08 PowerPoint Presentation ID4479101
Good Electrical Conductors When Dissolved In Water These compounds are \emph{molecular} and are not acids or bases. when we dissolve hydrogen chloride gas in water, we find that the solution is a very good conductor. Electrolytes may be covalent compounds that chemically react with water to produce ions (for. These compounds are \emph{molecular} and are not acids or bases. conductivity measurements reveal that most covalent compounds, if they dissolve in water at all, retain their original molecular structures. Salt contains nacl and kcl, which form electrolytes when dissolved in water, most of which become ions. The water molecules play an essential part in forming ions. A substance that, when added to water, renders it. Metals are good conductors of electricity because the delocalised electrons in the metal carry electrical charge through the metal. electrolytic solutions are those that are capable of conducting an electric current. Substances that dissolve in water to yield ions are called electrolytes. 4.2.2.8 metals as conductors. Most familiar is the conduction of electricity. to conduct electricity, a substance must contain freely mobile, charged species.
From www.numerade.com
SOLVED 'Why do ionic compounds make good conductors when dissolved in Good Electrical Conductors When Dissolved In Water Most familiar is the conduction of electricity. Salt contains nacl and kcl, which form electrolytes when dissolved in water, most of which become ions. electrolytic solutions are those that are capable of conducting an electric current. conductivity measurements reveal that most covalent compounds, if they dissolve in water at all, retain their original molecular structures. to conduct. Good Electrical Conductors When Dissolved In Water.
From www.vrogue.co
Chemical Bonding Electrical Conductivity Of Ionic Com vrogue.co Good Electrical Conductors When Dissolved In Water Electrolytes may be covalent compounds that chemically react with water to produce ions (for. 4.2.2.8 metals as conductors. to conduct electricity, a substance must contain freely mobile, charged species. A substance that, when added to water, renders it. The water molecules play an essential part in forming ions. These compounds are \emph{molecular} and are not acids or bases.. Good Electrical Conductors When Dissolved In Water.
From electricitytohitei.blogspot.com
Electricity Good Conductors Of Electricity Good Electrical Conductors When Dissolved In Water to conduct electricity, a substance must contain freely mobile, charged species. Salt contains nacl and kcl, which form electrolytes when dissolved in water, most of which become ions. Most familiar is the conduction of electricity. Electrolytes may be covalent compounds that chemically react with water to produce ions (for. conductivity measurements reveal that most covalent compounds, if they. Good Electrical Conductors When Dissolved In Water.
From www.slideserve.com
PPT Plan for Wed, 1 Oct 08 PowerPoint Presentation ID4479101 Good Electrical Conductors When Dissolved In Water A substance that, when added to water, renders it. Electrolytes may be covalent compounds that chemically react with water to produce ions (for. These compounds are \emph{molecular} and are not acids or bases. 4.2.2.8 metals as conductors. Salt contains nacl and kcl, which form electrolytes when dissolved in water, most of which become ions. Metals are good conductors of. Good Electrical Conductors When Dissolved In Water.
From slideplayer.com
Acids and Bases (P ) Lesson 12 September 14th, ppt download Good Electrical Conductors When Dissolved In Water The water molecules play an essential part in forming ions. Substances that dissolve in water to yield ions are called electrolytes. A substance that, when added to water, renders it. when we dissolve hydrogen chloride gas in water, we find that the solution is a very good conductor. 4.2.2.8 metals as conductors. Metals are good conductors of electricity. Good Electrical Conductors When Dissolved In Water.
From dewwool.com
Conductivity of water DewWool Good Electrical Conductors When Dissolved In Water Metals are good conductors of electricity because the delocalised electrons in the metal carry electrical charge through the metal. A substance that, when added to water, renders it. These compounds are \emph{molecular} and are not acids or bases. Most familiar is the conduction of electricity. when we dissolve hydrogen chloride gas in water, we find that the solution is. Good Electrical Conductors When Dissolved In Water.
From slidetodoc.com
Chemical effect of electric current How things work Good Electrical Conductors When Dissolved In Water Most familiar is the conduction of electricity. electrolytic solutions are those that are capable of conducting an electric current. when we dissolve hydrogen chloride gas in water, we find that the solution is a very good conductor. Substances that dissolve in water to yield ions are called electrolytes. Salt contains nacl and kcl, which form electrolytes when dissolved. Good Electrical Conductors When Dissolved In Water.
From learningschooltrkesp5v.z22.web.core.windows.net
Water Is Conductor Of Electricity Good Electrical Conductors When Dissolved In Water These compounds are \emph{molecular} and are not acids or bases. Most familiar is the conduction of electricity. 4.2.2.8 metals as conductors. The water molecules play an essential part in forming ions. electrolytic solutions are those that are capable of conducting an electric current. to conduct electricity, a substance must contain freely mobile, charged species. Substances that dissolve. Good Electrical Conductors When Dissolved In Water.
From www.teachoo.com
The table shown below gives information about four Chemistry MCQ Good Electrical Conductors When Dissolved In Water These compounds are \emph{molecular} and are not acids or bases. electrolytic solutions are those that are capable of conducting an electric current. The water molecules play an essential part in forming ions. Metals are good conductors of electricity because the delocalised electrons in the metal carry electrical charge through the metal. A substance that, when added to water, renders. Good Electrical Conductors When Dissolved In Water.
From slideplayer.com
Ch. 7 LT1 Ionic Bonding and Metals ppt download Good Electrical Conductors When Dissolved In Water when we dissolve hydrogen chloride gas in water, we find that the solution is a very good conductor. Most familiar is the conduction of electricity. Salt contains nacl and kcl, which form electrolytes when dissolved in water, most of which become ions. The water molecules play an essential part in forming ions. to conduct electricity, a substance must. Good Electrical Conductors When Dissolved In Water.
From www.youtube.com
Materials that are Good Conductor of Heat and Electricity YouTube Good Electrical Conductors When Dissolved In Water to conduct electricity, a substance must contain freely mobile, charged species. The water molecules play an essential part in forming ions. electrolytic solutions are those that are capable of conducting an electric current. Metals are good conductors of electricity because the delocalised electrons in the metal carry electrical charge through the metal. conductivity measurements reveal that most. Good Electrical Conductors When Dissolved In Water.
From printablelistparodic.z14.web.core.windows.net
Electricity Using Salt Water Good Electrical Conductors When Dissolved In Water These compounds are \emph{molecular} and are not acids or bases. conductivity measurements reveal that most covalent compounds, if they dissolve in water at all, retain their original molecular structures. Most familiar is the conduction of electricity. when we dissolve hydrogen chloride gas in water, we find that the solution is a very good conductor. Substances that dissolve in. Good Electrical Conductors When Dissolved In Water.
From www.youtube.com
Electrical conductivity with salt water YouTube Good Electrical Conductors When Dissolved In Water Metals are good conductors of electricity because the delocalised electrons in the metal carry electrical charge through the metal. Most familiar is the conduction of electricity. These compounds are \emph{molecular} and are not acids or bases. 4.2.2.8 metals as conductors. conductivity measurements reveal that most covalent compounds, if they dissolve in water at all, retain their original molecular. Good Electrical Conductors When Dissolved In Water.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at Good Electrical Conductors When Dissolved In Water Salt contains nacl and kcl, which form electrolytes when dissolved in water, most of which become ions. Substances that dissolve in water to yield ions are called electrolytes. electrolytic solutions are those that are capable of conducting an electric current. Most familiar is the conduction of electricity. These compounds are \emph{molecular} and are not acids or bases. to. Good Electrical Conductors When Dissolved In Water.
From www.youtube.com
electrical conductivity with salt water working model school science Good Electrical Conductors When Dissolved In Water Metals are good conductors of electricity because the delocalised electrons in the metal carry electrical charge through the metal. Most familiar is the conduction of electricity. conductivity measurements reveal that most covalent compounds, if they dissolve in water at all, retain their original molecular structures. 4.2.2.8 metals as conductors. electrolytic solutions are those that are capable of. Good Electrical Conductors When Dissolved In Water.
From abhyasonline.in
Concept Explanation Good Electrical Conductors When Dissolved In Water Most familiar is the conduction of electricity. Substances that dissolve in water to yield ions are called electrolytes. These compounds are \emph{molecular} and are not acids or bases. The water molecules play an essential part in forming ions. electrolytic solutions are those that are capable of conducting an electric current. Metals are good conductors of electricity because the delocalised. Good Electrical Conductors When Dissolved In Water.
From www.youtube.com
ELECTRICAL CONDUCTIVITY OF LIQUIDS YouTube Good Electrical Conductors When Dissolved In Water 4.2.2.8 metals as conductors. Substances that dissolve in water to yield ions are called electrolytes. A substance that, when added to water, renders it. to conduct electricity, a substance must contain freely mobile, charged species. The water molecules play an essential part in forming ions. Metals are good conductors of electricity because the delocalised electrons in the metal. Good Electrical Conductors When Dissolved In Water.
From www.youtube.com
Conductors and Insulators Science Experiment Good conductor and bad Good Electrical Conductors When Dissolved In Water The water molecules play an essential part in forming ions. when we dissolve hydrogen chloride gas in water, we find that the solution is a very good conductor. Most familiar is the conduction of electricity. Metals are good conductors of electricity because the delocalised electrons in the metal carry electrical charge through the metal. electrolytic solutions are those. Good Electrical Conductors When Dissolved In Water.